Trends in electronegativity down a group. For example the electronegativity trend across period 3 in the periodic table is depicted below.

Periodic Trends Made Easy Chemtalk
As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table.

. On the Periodic Table of elements electronegativity increases as you move left to right across a period. F g e- F- g energy Trends in EA Across a period. If it increases up to fluorine it must decrease as you go down The chart shows the patterns of electronegativity in Groups 1 and 7.
There are no units involved nor is it a property but is a relative scale from 1-4. The important periodic properties are atomic size metallic character non-metallic character ionization potential electron affinity and electronegativity. Periodic Table Trends.
This pattern usually begins with a Group I element and ending with a Group 8 element. Electronegativity increases from left to right of a row as across the row the number of protons in the nucleus increases. Which of the following trends is similar to electronegativity follows the same pattern.
There are certain trends and patterns in the way elements react and behave. The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right. What is period trend for electronegativity.
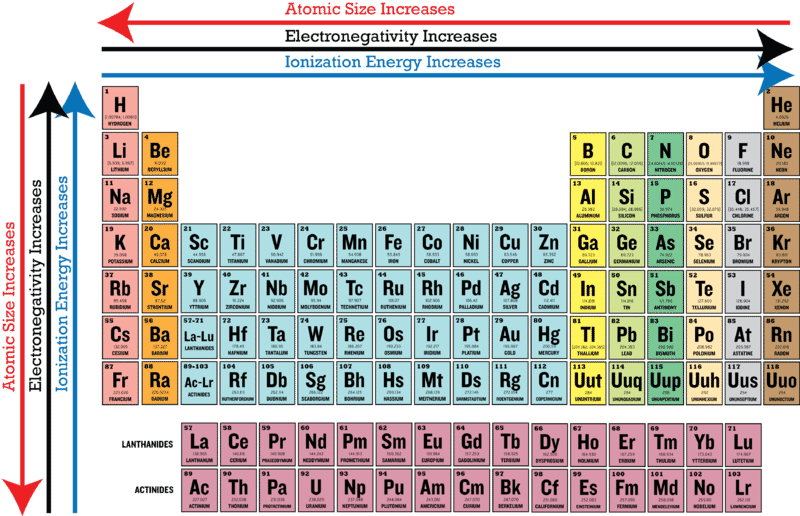
Electronegativities generally decrease from top to bottom of a group. As the atomic radius 2 increases and the number of completed electron shells energy levels increases going down the group the power of the atoms nucleus to attract electrons to. For example the format of the periodic table is designed so properties can be easily compared.
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Explain why the trend in electronegativity follows the pattern that it does as you move left to right across the periodic table. As you go down a group electronegativity decreases.
This trend is seen as you move across the periodic table from left to right. Does this agree with the trend in. This summary image is courtesy of WilenskyChemistry.
Therefore electronegativity increases across a period and decreases down a group. Down a Family 42. The periodic table affect the electronegativity.
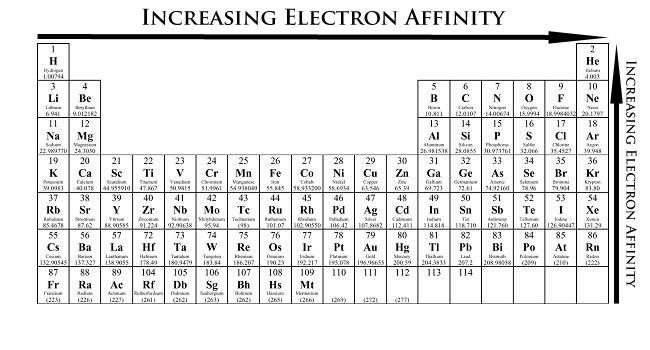
In general the electronegativities of the elements in a Group of the periodic table decrease as you go down the group from top to bottom. Electron Affinity The energy released when an electron is added to a gaseous atom Ag e-1 A-1 energy The atom turns into an anion in the process The process for fluorine can be represented as follows. The electronegativity trend refers to a trend that can be seen across the periodic table.
The trends of the electronegativity are. Lithium 10 and Francium 07 in Group I. Periodic trends are specific patterns in the properties of chemical elements that are revealed in the periodic table of elements.
Lithium 10 and Fluorine 40 in period 2. People also asked Of elements in. This trend in properties is known as periodic properties.
The trends for electronegativity is that the value increases across the periods rows of the periodic table. 120 rows We observe a common trend in properties as we move across a period from left to right or down the group. Electronegativity refers to an atoms ability to attract electrons in a chemical bond.
We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine. Decrease in a group down and increase in a period from left to right. This is because there is more electron-attracting power of the nucleus with the increasing nuclear charge as the number of protons increase form left to right across the periodic table.
The electronegativity also increases up a group column of the periodic table. How does the increase in the number of protons as you move down the family affect the electronegativity. Trends in Electronegativities in Groups of the Periodic Table.
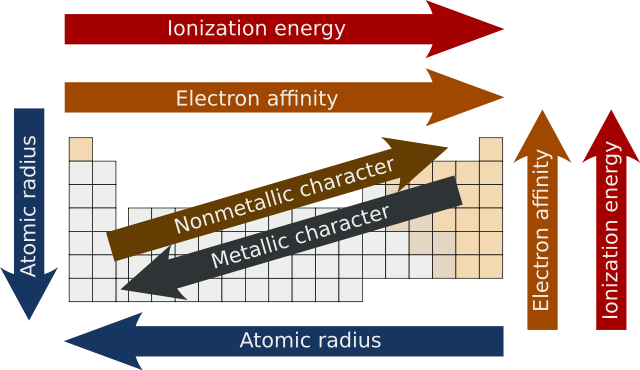
So electronegativity increases up a group and across the row of the periodic table. The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. In this graph we have not shown argon as it does not react with elements to form bonds.
The Periodic Law states that when elements are arranged in order of increasing atomic number their physical and chemical properties show a periodic pattern. A period number is the number that is given to a group of elements across the periodic table that have made a round from completing its outer electron shell. The periodic trends in properties of elements.
The Periodic Table is arranged according to Periodic Law. When we move from left to right in a period of the modern periodic table electronegativity increases. Trends in electronegativity across a period.
There is an increased nuclear attraction AND the elements on the right side of. The trend closely resembles electronegativity Increases as you move left to right across a period cokephysicsuclaedulaptag mchselectronegativity-sjpg. Since the electronegativity of some of.
This allows a stronger attraction between outermost electrons and the nucleus. Electronegativity follows the same pattern as ionization energy and the opposite pattern of atomic radius. Electronegativity increases as you go from left to right across a period.
The electronegativity increases while it decreases as you move down a group of elements. Major periodic trends include electronegativity ionization energy electron affinity atomic radii ionic radius metallic character and chemical reactivity. 9th - 12th grade.
These patterns can be discovered by examining the changes in properties of elements on the Periodic Table. Electronegativity values generally increase from left to right across the periodic table. The highest electronegativity value is for fluorine.
So for example period I would be from hydrogen to helium. Explaining the patterns in electronegativity. Increasing e- affinity Reason.
Periodic Trends Chemistry Libretexts

Periodic Trends Made Easy Chemtalk
Periodic Trends In Electronegativity Ck 12 Foundation
What Trend In Electronegativity Do You See As You Go Across A Period Row On The Periodic Table Socratic

What Is Electronegativity Trends Chart Periodic Table Chemtalk


0 comments
Post a Comment